CN Lewis Structure - A Simple Guide
Ever wonder how chemists figure out what a molecule looks like, or how its tiny pieces fit together? It's a bit like putting together a puzzle, where each atom needs its right spot and its right number of connections. One really helpful way to picture these tiny arrangements is by drawing what are called Lewis structures, and they show us where all the shared and unshared electrons hang out. This way of seeing things, you know, it helps a lot with getting a better grip on how a molecule behaves and what its true shape might be.
These drawings are a neat trick for seeing the invisible, for instance, how atoms like carbon and nitrogen come together to form things like the cyanide ion, or even neutral CN, and the cyanonium ion. They help us count up all the electron pairs that are making connections, and the ones that are just sitting there, waiting, as a matter of fact. When we talk about the cyanide ion, for example, its Lewis dot drawing starts with a carbon atom linked to a nitrogen atom using three lines, which represent shared electron pairs.
Then, on the outside of each atom, you will find two little dots. These dots are there to show us the electrons that aren't shared with another atom, the ones that are just hanging out by themselves. Knowing how to put these pieces onto paper can make a big difference in how you think about these tiny building blocks, and how they arrange themselves, pretty much. It's a fundamental skill, really, for anyone wanting to get a closer look at the very small world of chemical connections.
- Wilson Last Name Origin
- Dead Rising Characters
- Billie Eilish Cover
- Christina Ricci As Wednesday Addams
- Is Jenni Rivera Alive 2025
- What Are Lewis Structures, Anyway?
- Getting Started - The Basics of Drawing
- Counting Valence Electrons - Why Does It Matter for CN Lewis Structure?
- Drawing the Cyanide Ion - CN- Lewis Structure
- What About the Neutral CN Lewis Structure?
- How Do We Draw the Cyanonium Ion - CN+ Lewis Structure?
- Adding Lone Pairs and Charges - The Final Touches
- A Closer Look at Bonds in CN Lewis Structure
What Are Lewis Structures, Anyway?
Lewis structures are a way of drawing molecules that shows us where the electrons are located around the atoms. It's a simple picture, in a way, that helps us figure out how atoms are connected and where the unshared electron pairs, sometimes called lone pairs, might be sitting. Think of it like a map for the electrons, telling you which ones are involved in making connections between atoms and which ones are just chilling out on one atom by itself. This visual aid, you know, it makes it easier to get a sense of the electron arrangement.
- Cast Of The Long Walk Home
- Krispy Kreme Releases Limited Edition Pac Man Themed Doughnuts
- Elvis And Ginger
- Eddie On Desperate Housewives
- Jack Wagner Bold And Beautiful Return
This kind of drawing was thought up by a person named Lewis, and his idea was to give us an electron dot picture of a molecule. This picture is quite useful for getting a better grasp of what a molecule is all about, and it helps us find out about all the pairs of electrons that are shared between atoms, which are called bond pairs, and all the pairs of electrons that are just on their own, which are called lone pairs, present with each atom. So, it's a tool for seeing the unseen, almost, in the world of atoms and their connections.
When you look at one of these drawings, you'll see symbols for the atoms, like 'C' for carbon or 'N' for nitrogen. Then, you'll notice lines or dashes between them, which are there to represent the shared electron pairs, the ones forming the connections. And then there are dots, usually in pairs, placed around the atom symbols, which are there to show the electrons that are not shared with any other atom, just belonging to that one atom. This system, it really helps to make the invisible parts of a molecule a little more visible, you know, to our minds.
Getting Started - The Basics of Drawing
Starting to draw a Lewis structure for any molecule means you need to do a little bit of counting first. You see, every atom has what are called valence electrons, and these are the electrons that are on the very outside shell of the atom. They're the ones that actually get involved in making connections with other atoms, or just sitting there as lone pairs. So, the first move is to figure out the total count of these valence electrons for every single atom in the molecule you are looking at, as a matter of fact.
For example, if you were going to figure out the Lewis structure for something like CH3C(O)CN, you would begin by adding up all the valence electrons from each atom in that whole setup. Carbon typically brings four valence electrons to the table, while hydrogen brings one, and oxygen usually has six, and nitrogen, well, nitrogen usually has five. You'd sum all those up to get a grand total, and that total number is what you have to work with when you start placing electrons around the atoms in your drawing, more or less. It's the starting point for everything.
Once you have that total number, you can then begin to place the atoms on your drawing space, usually with the least electronegative atom in the middle, though for simple ones like CN, it's pretty straightforward. Then, you start connecting them with single lines, which each stand for two shared electrons. After that, you distribute the remaining electrons as lone pairs around the outside atoms first, and then the central atom, making sure each atom gets its full set of eight electrons, if possible, which is called an octet. This process, it just helps to organize your thoughts about how the electrons are arranged.
Counting Valence Electrons - Why Does It Matter for CN Lewis Structure?
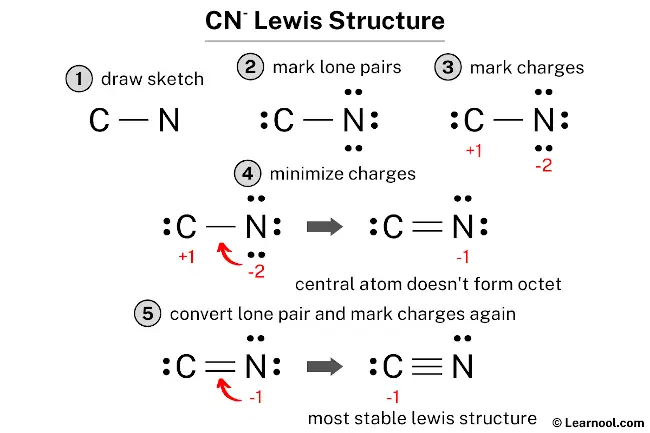
Counting the valence electrons is, you know, a really big deal when you're trying to figure out a Lewis structure. These are the electrons that are on the very outer part of an atom, and they are the ones that actually do the work of making connections or just hanging out by themselves. For something like the cyanide ion, which is written as CN-, knowing this count is absolutely necessary for getting the drawing right. If you don't get the total number correct at the beginning, then the whole picture you draw will be off, pretty much.
Let's take the cyanide ion, CN-, as an example. Carbon, if you look at it on the periodic table, usually has four valence electrons. Nitrogen, on the other hand, typically has five valence electrons. But because this is an ion with a negative charge, CN-, that means it has gained one extra electron. So, you add that extra electron to your count. That makes it four from carbon, plus five from nitrogen, plus one extra electron from the negative charge, which gives you a grand total of ten valence electrons for the cyanide ion. That number, you see, is what you need to keep track of as you draw.
This total of ten valence electrons is what you will use to form all the connections and place all the lone pairs in the Lewis structure for CN-. It's like having a set number of building blocks, and you have to use all of them, and only them, to build your structure. If you had, say, nineteen electrons, or twenty, or even just two, the structure would look entirely different, and it would represent a completely different chemical thing. So, getting this first step right, it's really quite important for the whole process.
Drawing the Cyanide Ion - CN- Lewis Structure
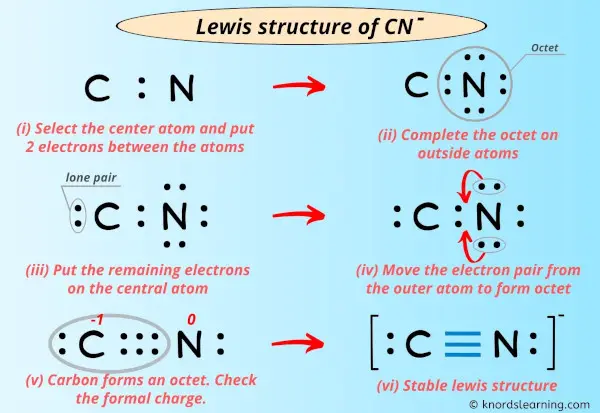
When you are ready to draw the Lewis structure for the cyanide ion, CN-, you've already figured out that it has ten valence electrons in total. The instructions tell us that the drawing for the cyanide ion begins with a carbon atom connected to a nitrogen atom with three dashes. These three dashes, in fact, mean that there are three shared pairs of electrons between the carbon and nitrogen atoms, forming what we call a triple connection. This is a very strong connection, by the way.
So, you would put down a 'C', then draw three lines right next to it, and then put down an 'N' on the other side of those lines. Each line represents two electrons, so those three lines account for six of your ten total valence electrons. That leaves you with four electrons that you still need to place in your drawing. The instructions then say that on the opposite sides of each atom, there should be two dots for the unshared valence electrons. This means two dots on the carbon atom and two dots on the nitrogen atom.
If you put two dots on the carbon and two dots on the nitrogen, that uses up the remaining four electrons (two plus two equals four). Now, let's check if each atom has a full outer shell, meaning eight electrons around it, which is the general rule. For the carbon atom, it has six electrons from the triple connection and two electrons from its lone pair, making a total of eight. For the nitrogen atom, it also has six electrons from the triple connection and two electrons from its lone pair, giving it eight as well. So, this arrangement, you know, works out pretty well for both atoms.
What About the Neutral CN Lewis Structure?
Now, let's think about the neutral CN molecule, without any charge. This is a bit different from the cyanide ion because it doesn't have that extra electron. For neutral CN, carbon still brings four valence electrons, and nitrogen still brings five valence electrons. So, if you add those up, you get a total of nine valence electrons. This is an odd number, which means it's not going to be able to form neat pairs for everything, which is that, a bit unusual for stable molecules.
With nine electrons, you can't give every atom a full set of eight electrons, an octet, without leaving one electron unpaired. You could try to make a triple connection between carbon and nitrogen, like in the ion, which would use six electrons. That leaves three electrons. If you put one lone pair on carbon and one lone pair on nitrogen, that uses four electrons, which is too many. If you put one lone pair on carbon and one electron on nitrogen, or vice versa, it still leaves an odd electron. This means that one of the atoms will end up with an odd number of electrons, making it what's called a radical. This kind of molecule, you know, tends to be very reactive because of that single, unpaired electron.
So, for neutral CN, you would still likely have a triple connection between the carbon and nitrogen. Then, you'd place a lone pair on one atom, let's say nitrogen, and a single, unpaired electron on the carbon atom. This arrangement gives nitrogen eight electrons around it (six from the triple connection, two from the lone pair), and carbon would have seven electrons (six from the triple connection, one unpaired electron). This makes it a very interesting molecule to study, in some respects, because of that odd electron.
How Do We Draw the Cyanonium Ion - CN+ Lewis Structure?
Moving on to the cyanonium ion, which is CN+, things change again because of the positive charge. A positive charge means that the molecule has lost an electron. So, for CN+, you start with the usual four valence electrons from carbon and five from nitrogen. But then, you subtract one electron because of that positive charge. This gives you a total of eight valence electrons to work with for the cyanonium ion. That's a pretty different number compared to the ten for CN- or the nine for neutral CN.
With eight electrons, you're looking to make connections and place lone pairs so that each atom, if possible, ends up with eight electrons around it. If you try a triple connection between carbon and nitrogen, that uses six electrons. That leaves you with two electrons remaining. You could place those two electrons as a lone pair on one of the atoms. For instance, you could put them on the nitrogen atom. This would give nitrogen six electrons from the triple connection and two from the lone pair, making eight. But then carbon would only have six electrons from the triple connection, which is not a full octet.
Alternatively, you could try a double connection between carbon and nitrogen, which would use four electrons. That would leave you with four electrons to place as lone pairs. You could put two electrons as a lone pair on carbon and two electrons as a lone pair on nitrogen. In this case, carbon would have four from the double connection and two from its lone pair, making six. Nitrogen would have four from the double connection and two from its lone pair, also making six. Neither atom has a full octet in this setup, which is, you know, not ideal. The most stable arrangement for CN+ typically involves a triple connection and then formal charges to show where the positive charge really sits, usually on the carbon atom. This means a lone pair on nitrogen, and a positive charge on carbon, which has only six electrons around it, making it electron-deficient, basically.
Adding Lone Pairs and Charges - The Final Touches
Once you've drawn the connections, whether they are single, double, or triple, the next very important step is to add in all the lone pairs of electrons. These are the electrons that are not involved in making connections between atoms, but they are still part of the atom's outer shell. For the cyanide ion, CN-, as we discussed, after putting in the triple connection between carbon and nitrogen, you had four electrons left. You then place two dots on the carbon atom and two dots on the nitrogen atom, showing those unshared valence electrons. This makes sure each atom has its complete set of eight electrons, which is a common goal for many atoms in molecules, in fact.
After placing all the lone pairs, you also need to think about charges. If the molecule you are drawing is an ion, like CN- or CN+, then you need to show that overall charge on your drawing. For CN-, you would draw the entire structure inside brackets and put a negative sign outside the top right corner of the brackets. This indicates that the whole structure has an extra electron, giving it a negative charge. This is a pretty standard way of doing things in chemistry, to be honest.
For CN+, you would do something similar, but with a positive sign outside the brackets. Sometimes, you also need to figure out what are called "formal charges" on individual atoms within the molecule. This helps you understand where the overall charge is "located" or distributed. For CN- with its triple connection and lone pairs, the formal charge on carbon is -1 and on nitrogen is 0, which sums up to the overall -1 charge. For CN+, the formal charge on carbon would be +1 and on nitrogen 0, summing to +1. This helps to show the true electron distribution, you know, in a more complete way.
A Closer Look at Bonds in CN Lewis Structure
When we talk about bonds in a Lewis structure, we are really talking about the shared pairs of electrons that hold atoms together. These can be single bonds, where two electrons are shared; double bonds, where four electrons are shared; or triple bonds, where six electrons are shared. The number of bonds present in a molecule, especially for something like the cyanide ion, is a key piece of information that the Lewis structure helps us figure out. It's a fundamental aspect of how these atoms stick together, actually.
For the cyanide ion, CN-, the drawing we discussed has a carbon atom connected to a nitrogen atom with three dashes. Each dash represents a pair of shared electrons, so three dashes mean there are three pairs of electrons being shared. This is what we call a triple bond. So, the answer to how many bonds are present in its Lewis structure is three. This triple bond is a very strong connection, making the cyanide ion quite stable in many situations, more or less.
Understanding the type of bond, whether it's single, double, or triple, also tells you something about the distance between the atoms and the strength of the connection. Triple bonds are shorter and stronger than double bonds, which are in turn shorter and stronger than single bonds. So, just by looking at the Lewis structure for CN-, you can tell that the carbon and nitrogen atoms are held together very tightly. This kind of detail, you know, is really helpful for predicting how a molecule might behave in different chemical reactions.
CN- Lewis structure - Learnool
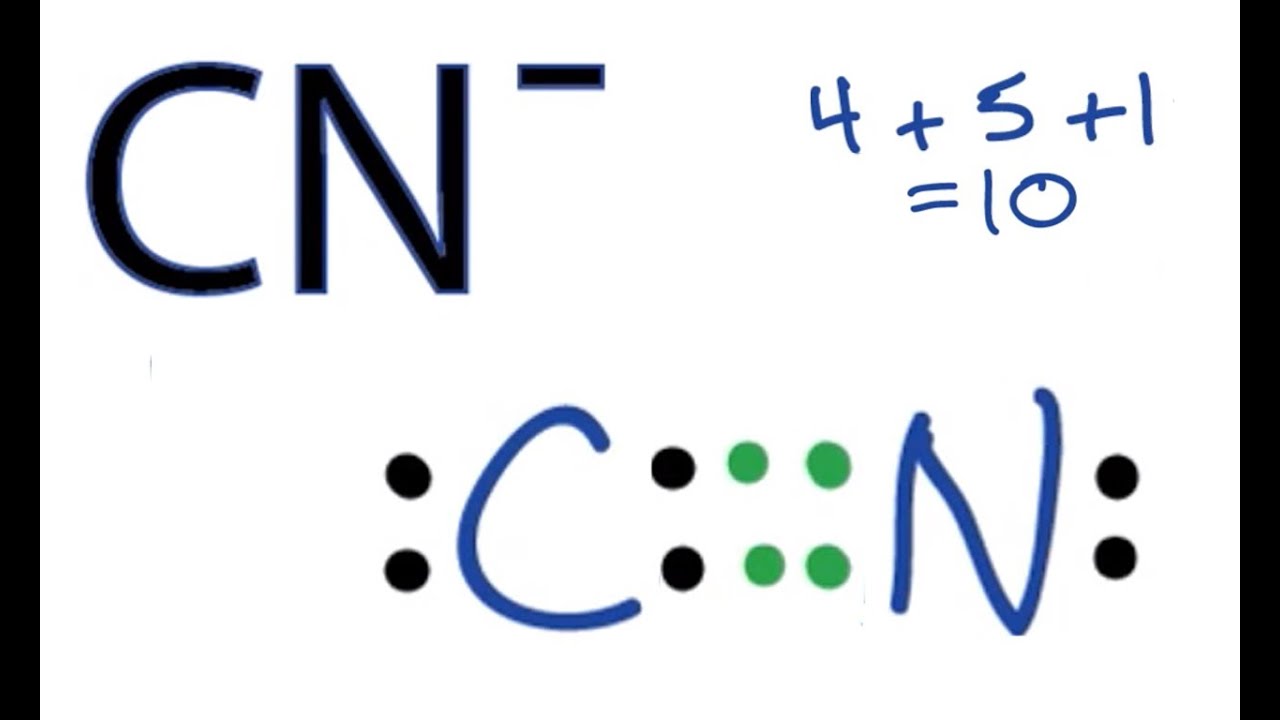
CN- Lewis Structure: How to Draw the Dot Structure for the CN- - YouTube
Lewis Structure of CN- (With 6 Simple Steps to Draw!)